The educational module
For each of these stages of the biomarker value chain, the fact sheets propose:
- a short description of key requirements to address the next stage
- the questions to be listed, the issues to be taken into consideration or the selection criteria
-
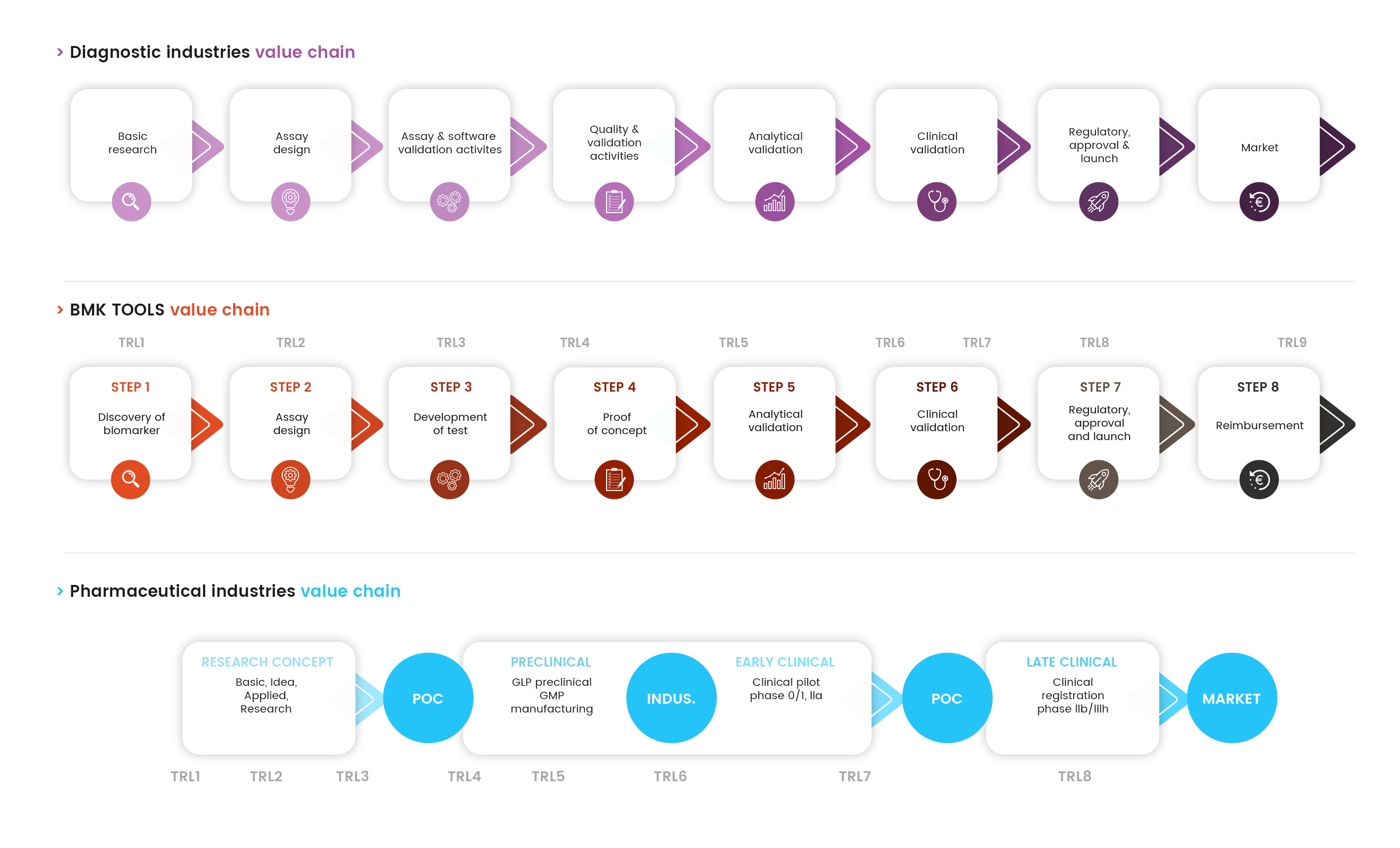
Step 1
Discovery of biomarker
Step 1 - Discovery of biomarkerThe first step of the biomarker value chain is the discovery of the biomarker. During this step, the biomarker is identified, and the basic principles are described. A review of the scientific literature, patents, existing technologies, the pathologies concerned is important to fully characterise the biomarker. This includes a description of the nature, type of biomarker and its potential use in the clinic.
-
Step 2
Assay design
Step 2 - Assay designAt this stage, it is a matter of generating ideas, hypotheses and experimental plans for the design of the diagnostic test used to detect the candidate biomarker. During the development of a test, a comprehensive reflection on the biomarker and its test in the early stages of development allows the formulation of hypotheses and experimental plans in line with the possibilities for use in common practice.
-
Step 3
Development of test
Step 3 - Development of testAt this stage, it is a matter of demonstrating the functionality of the diagnostic test and to provide experimental proof that this test makes it possible to detect the biomarker candidate from simple samples, in laboratory conditions.
-
Step 4
Proof of concept
Step 4 - Proof of conceptThe proof of concept stage is a critical step to ensure the next steps of the proper development of a diagnostic test. The objective of this stage is to determine whether the test is useable in practice.
-
Step 5
Analytical validation
Step 5 - Analytical validationThe analytical validation consists of the evaluation of the technical performance of the test. The objective at this stage is to demonstrate the robustness and quality of the test in terms of precision, specificity, sensitivity and reproducibility under practical conditions of use, on samples representative of the targeted population.
-
Step 6
Clinical validation
Step 6 - Clinical validationThe clinical validation stage is a very important one (in terms of time, investment) and may be broken down into several steps: clinical verification and clinical validation itself.
-
Step 7
Regulatory, approval and launch
Step 7 - Regulatory, approval and launchCE marking allows in vitro diagnostic medical devices (IVD MD) to be placed on the market in all countries of the European Union (EU). It is up to the legal manufacturer to affix the CE mark on its product once it is in full conformity with the basic requirements relating to quality, security and device performance as defined in the IVD Directive (98/79/CE).
-
Step 8
Reimbursement
Step 8 - ReimbursementThroughout diagnostic development, testing and application, all steps of the product elaboration research and development can influence reimbursement and market access strategies. While most organizations start developing their approach to target markets once the diagnostic has obtained CE marking, proactive examination of requirements and reimbursement/market access pathways assists in having the test fit the system, which in turn speeds up reimbursement.
Download
What is the correlation between the value chain
and the TRL (Technology readiness level)?
Patent application, which constitutes a key moment during this development path, needs to be addressed with the valorisation structures ad hoc. Nevertheless, in order to qualify the development stage of the project in particular when looking for funds and/or partnerships, a scale of technological readiness (the TRL scale, Technology Readiness Level) adapted to (bio)drugs is proposed. Furthermore, the corresponding levels of development are shown in the different fact sheets. The correspondence with the BMK TOOLS® value chain is represented below.